
Na-S or Sodium-Sulfur Battery
The Sodium-Sulfur battery is composed of a solid electrolyte membrane between its anode and cathode. Due to very high energy efficiency, Sodium-Sulphur battery finds applications in grid energy storage and space explorations. In structure, the Sodium – Sulfur battery is cylindrical in shape and is enclosed in a steel case coated with Chromium

Sodium-Sulfur (NAS Battery
Sodium-Sulfur NAS® NAS battery can provide effective solutions to any issues due to huge introduction of renewable energy on transmission & distribution grids in India. Recommendations: 1)Recognizing battery for grid application as an essential infrastructure for realizing

Stable all-solid-state sodium-sulfur batteries for low-temperature
Sodium-sulfur (Na-S) batteries with sodium metal anode and elemental sulfur cathode separated by a solid-state electrolyte (e.g., beta-alumina electrolyte) membrane have been utilized practically in stationary energy storage systems because of the natural abundance and low-cost of sodium and sulfur, and long-cycling stability [1], [2].Typically, Na-S batteries

Here''s What You Need to Know About Sodium Sulfur (NaS)
The sodium sulfur battery is a megawatt-level energy storage system with high energy density, large capacity, and long service life. Learn more. Call +1(917) 993 7467 or connect with one of our experts to get full access to the most comprehensive and verified construction projects happening in

Sodium Sulfur Battery
In the sodium–sulfur battery, the active materials sodium and sulfur are in the liquid state under operating conditions. Upon discharge, Na 2 S 5 is formed initially and is subsequently reduced to polysulfides of composition Na 2 S x (2.7<x<5), which are also in the liquid phase. The theoretical cell voltage amounts to 2.076 V. The following

Sodium-Sulfur Batteries with a Polymer-Coated NASICON-type Sodium
The two anodic waves are related to the transition of sodium sulfide and/or low-order sodium polysulfides to high-order sodium polysulfide species and further to elemental sulfur. Figure 3 D presents the charge/discharge profiles of the Na ǁ PIN-Na 3 Zr 2 Si 2 PO 12 ǁ CNF/S cells operated at a variety of C rates.

Frontiers for Room-Temperature Sodium–Sulfur Batteries
Room-temperature (RT) sodium–sulfur (Na-S) systems have been rising stars in new battery technologies beyond the lithium-ion battery era. This Perspective provides a glimpse at this technology, with an emphasis on discussing its fundamental challenges and strategies that are currently used for optimization. We also aim to systematically correlate the functionality of

Progress and prospects of sodium-sulfur batteries: A review
A commercialized high temperature Na-S battery shows upper and lower plateau voltage at 2.075 and 1.7 V during discharge [6], [7], [8].The sulfur cathode has theoretical capacity of 1672, 838 and 558 mAh g − 1 sulfur, if all the elemental sulfur changed to Na 2 S, Na 2 S 2 and Na 2 S 3 respectively [9] bining sulfur cathode with sodium anode and suitable electrolyte

Achieving High-Performance Room-Temperature Sodium–Sulfur
Despite the high theoretical capacity of the sodium–sulfur battery, its application is seriously restrained by the challenges due to its low sulfur electroactivity and accelerated shuttle effect, which lead to low accessible capacity and fast decay. Herein, an elaborate carbon framework, interconnected mesoporous hollow carbon nanospheres, is reported as an effective

Sodium Sulfur Battery Market Key Findings by 2031
Sodium sulfur battery has a high density of energy, high charging or discharge efficiency, and a long life cycle. This type of battery is made from inexpensive materials. In a sodium-sulfur battery system, this type of battery is integrated into an energy storage system based on electrochemical charge/discharge reactions between a positive

The sodium sulfur battery (Book) | ETDEWEB
@misc{etde_5419869, title = {The sodium sulfur battery} author = {Sudworth, J L, and Tilley, A R} abstractNote = {The discovery of the sodium sulfur battery in the 1960''s was hailed by battery technologists around the world as the answer to storing electricity in a cheap and convenient way. This critical review distils the essence of nearly two decades of work from laboratories around
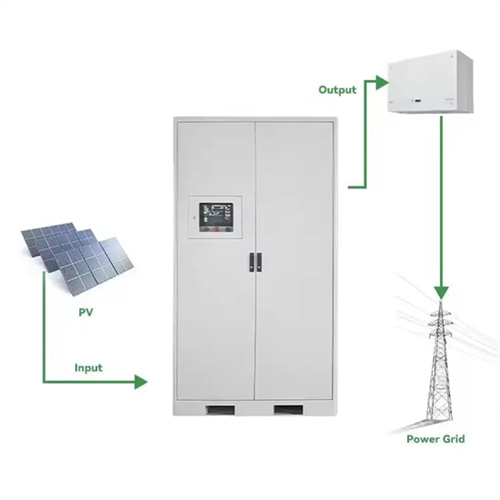
Technology Strategy Assessment
with the sodium-sulfur (NaS) battery as a potential temperature power source high- for vehicle electrification in the late 1960s [1]. The NaS battery was followed in the 1970s by the sodium-metal halide battery (NaMH: e.g., sodium-nickel chloride), also known as the ZEBRA battery (Zeolite Battery Research Africa Project or, more recently, Zero

Recent advances in electrolytes for room-temperature sodium-sulfur
Room temperature sodium-sulfur (RT Na–S) battery is an emerging energy storage system due to its possible application in grid energy storage and electric vehicles. In this review article, recent advances in various electrolyte compositions for RT Na–S batteries have been highlighted along with discussion on important aspects of using

Review on suppressing the shuttle effect for room-temperature sodium
Fig. 1 illustrates the chronological milestones in the development of various components of RT Na-S batteries. As early as 2006, the research model of RT Na-S batteries was initially proposed by Park et al [7] the subsequent period, the lack of reporting on certain research works makes it difficult to attract wide attention from the scientific community, which

Achieving High-Performance Room-Temperature
Despite the high theoretical capacity of the sodium–sulfur battery, its application is seriously restrained by the challenges due to its low sulfur electroactivity and accelerated shuttle effect, which lead to low

NAS Batteries | Products | NGK INSULATORS, LTD.
The NAS battery is a megawatt-level energy storage system that uses sodium and sulfur. The NAS battery system boasts an array of superior features, including large capacity, high energy density, and long service life, thus enabling a high output of electric power for long periods of time.

Sodium–Sulfur Flow Battery for Low-Cost Electrical Storage
A new sodium–sulfur (Na–S) flow battery utilizing molten sodium metal and flowable sulfur-based suspension as electrodes is demonstrated and analyzed for the first time. Unlike the conventional flow battery and the high-temperature Na–S battery, the proposed flow battery system decouples the energy and power thermal management by

A Critical Review on Room‐Temperature Sodium‐Sulfur
Sodium (Na) element accounts for 2.36% of the earth''s crust and can be easily harvested from sea water, while sulfur (S) is the 16th most abundant element on earth with high production of 70 million tons per year. The combination of Na and S into RT-Na/S batteries represents an ideal choice of battery with an affordable low material price.

Sodium Batteries: A Review on Sodium-Sulfur and Sodium-Air Batteries
Lithium-ion batteries are currently used for various applications since they are lightweight, stable, and flexible. With the increased demand for portable electronics and electric vehicles, it has become necessary to develop newer, smaller, and lighter batteries with increased cycle life, high energy density, and overall better battery performance. Since the sources of

Long-life sodium–sulfur batteries enabled by super-sodiophilic
Sodium–metal batteries (SMBs) are an appealing sustainable low-cost alternative to lithium–metal batteries due to their high theoretical capacity (1165 mA h g−1) and abundance of sodium. However, the practical viability of SMBs is challenged by a non-uniform deposition and uncontrollable growth of dendrites

Intercalation-type catalyst for non-aqueous room temperature sodium
Li, S. et al. High-performance room temperature sodium–sulfur battery by eutectic acceleration in tellurium-doped sulfurized polyacrylonitrile. ACS Appl. Energy Mater. 2, 2956–2964 (2019).

Challenges and prospects for room temperature solid-state sodium-sulfur
Room temperature sodium-sulfur (Na-S) batteries, known for their high energy density and low cost, are one of the most promising next-generation energy storage systems. However, the polysulfide shuttling and uncontrollable Na dendrite growth as well as safety issues caused by the use of organic liquid electrolytes in Na-S cells, have severely hindered their

A Critical Review on Room‐Temperature
Among the various battery systems, room-temperature sodium sulfur (RT-Na/S) batteries have been regarded as one of the most promising candidates with excellent performance-to-price ratios. Sodium (Na) element accounts for

Research on Wide-Temperature Rechargeable Sodium-Sulfur
The high theoretical capacity (1672 mA h/g) and abundant resources of sulfur render it an attractive electrode material for the next generation of battery systems [].Room-temperature Na-S (RT-Na-S) batteries, due to the availability and high theoretical capacity of both sodium and sulfur [], are one of the lowest-cost and highest-energy-density systems on the

Unconventional Designs for Functional Sodium-Sulfur
Sodium-sulfur (Na–S) batteries that utilize earth-abundant materials of Na and S have been one of the hottest topics in battery research. The low cost and high energy density make them promising candidates for next

Spanish researchers reveal sodium and sulphur battery
Researchers at the University of Córdoba have developed a battery composed of sodium and sulphur that can be charged and discharged more than 2,000 times. Sulphur has replaced all toxic metals in the cathode, while lithium has been replaced by sodium in the anode. Image: University of Cordoba. By Carrie Hampel.

A novel one‐step reaction sodium‐sulfur battery with high areal sulfur
Room temperature sodium-sulfur (RT Na-S) batteries are gaining extensive attention as attractive alternatives for large-scale energy storage, due to low cost and high abundancy of sodium and sulfur in nature.

Sodium Sulphur Battery
A unique reference book which contains a critical review of the history and development of the sodium sulphur battery; a theoretical basis for its operation; and a very good survey of design techniques and performance. There are numerous excellent drawings and illustrations.

Structural regulation of electrocatalysts for room-temperature
1 天前· Room-temperature sodium–sulfur (RT Na–S) batteries have been regarded as promising energy storage technologies in grid-scale stationary energy storage systems due to their low

Top 10 Companies in Sodium Sulfur Battery Market in 2024
A Sodium Sulfur (NaS) battery is a high-temperature energy storage device that uses molten sodium as the anode and molten sulfur as the cathode, separated by a solid ceramic electrolyte. Known for its high energy density, long cycle life, and efficiency, the NaS battery is ideal for grid-scale energy storage, renewable energy integration, and

A Critical Review on Room‐Temperature
Sodium (Na) element accounts for 2.36% of the earth''s crust and can be easily harvested from sea water, while sulfur (S) is the 16th most abundant element on earth with high production of 70 million tons per year. The combination of Na
6 FAQs about [Afghanistan sodium sulphur battery]
What is a sodium sulfur battery?
A sodium–sulfur (NaS) battery is a type of molten-salt battery that uses liquid sodium and liquid sulfur electrodes. This type of battery has a similar energy density to lithium-ion batteries, and is fabricated from inexpensive and low-toxicity materials.
Are sodium-sulfur batteries suitable for energy storage?
This paper presents a review of the state of technology of sodium-sulfur batteries suitable for application in energy storage requirements such as load leveling; emergency power supplies and uninterruptible power supply. The review focuses on the progress, prospects and challenges of sodium-sulfur batteries operating at high temperature (~ 300 °C).
Can sodium-sulfur batteries operate at high temperature?
The review focuses on the progress, prospects and challenges of sodium-sulfur batteries operating at high temperature (~ 300 °C). This paper also includes the recent development and progress of room temperature sodium-sulfur batteries. 1. Introduction
Are ambient-temperature sodium-sulfur batteries a viable alternative to lithium-ion batteries?
Ambient-temperature sodium-sulfur (Na-S) batteries are potential attractive alternatives to lithium-ion batteries owing to their high theoretical specific energy of 1,274 Wh kg −1 based on the mass of Na 2 S and abundant sulfur resources. However, their practical viability is impeded by sodium polysulfide shuttling.
Is short-chain sulfur suitable for efficient sodium–sulfur batteries?
Xiao, F.P., Wang, H.K., Xu, J., et al.: Generating short-chain sulfur suitable for efficient sodium–sulfur batteries via atomic copper sites on a N, O-codoped carbon composite.
Are room-temperature sodium sulfur (RT-na/S) batteries a good choice?
Among the various battery systems, room-temperature sodium sulfur (RT-Na/S) batteries have been regarded as one of the most promising candidates with excellent performance-to-price ratios.